Technology

A major building block of our proprietary glycoengineering platforms, low-sugar vaccinations, originated from the pioneering work led by Professor Chi-Huey Wong, former President of Academia Sinica in Taiwan. It was recognized by IUPAC in 2023 as one of the Top 10 Emerging technologies in Chemistry, along with other transformative technologies such as artificial muscles and ChatGPT language models in Chemistry (https://iupac.org/iupac-2023-top-ten/). The approach is grounded in the well-established understanding that viruses use surface glycosylation as a common way to evade host immune responses, thereby hindering immune recognition and diminishing the efficacy of vaccines and antibodies. By eliminating the sugar coat from the surface antigen, our Low-Sugar Universal vaccine reveals conserved epitopes and induces an enhanced and broadly protective immune response. This concept is now increasingly being incorporated into vaccine designs and has resulted in a number of very promising vaccine development projects against multiple viral pathogens here at Rock Bio and elsewhere.With the rise of mRNA vaccines in recent years, we have expanded our low sugar vaccines to include both protein and mRNA platforms. While the two differ greatly in methodology, they share the same operational goal, that is, to sufficiently expose conserved epitopes while preserving the overall structure of the antigen proteins.
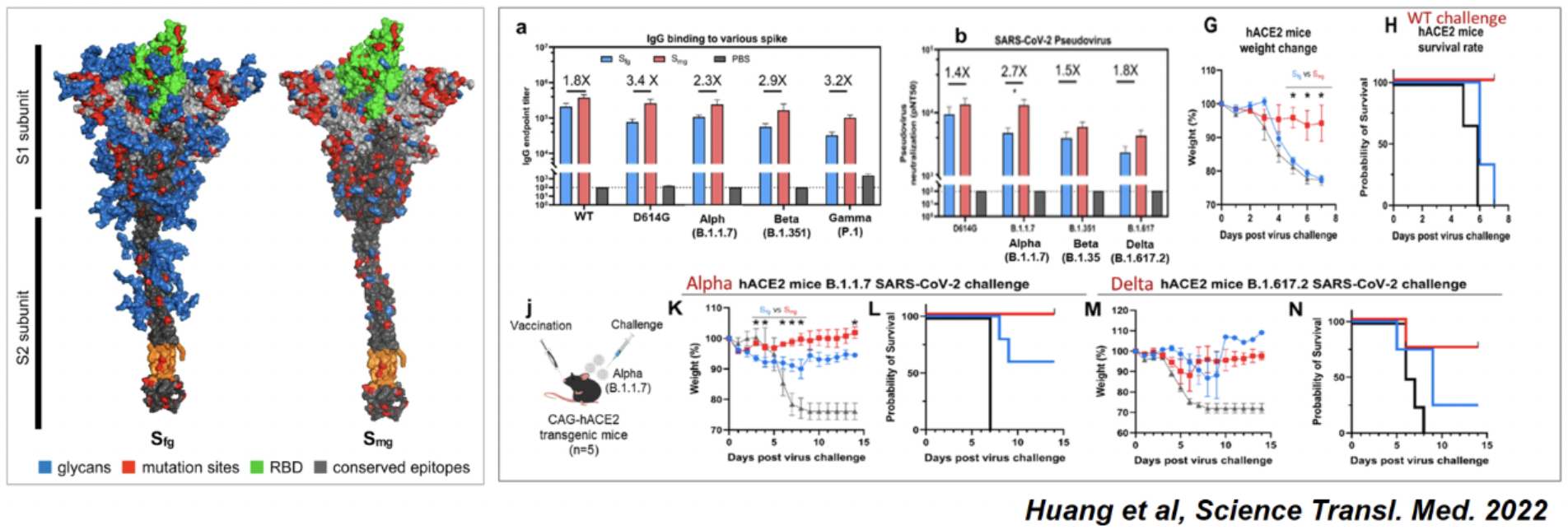
Our first low-sugar universal vaccine program, RBM001, is a glycan depleted SARS-CoV-2 Spike protein subunit vaccine. In animal models, this vaccine demonstrated broad protection against variants including alpha, delta (Huang et al, Science Transl. Med. 2022) and Omicron (unpublished). Over the last two years or so, the program successfully went through the various stages of clinical manufacturing and IND preparation. Its IND (investigational new drug) application has recently been approved by the US FDA and the Phase 1 clinical trial is now underway in the US (https://ichgcp.net/clinical-trials-registry/NCT06878170).
During the COVID-19 pandemic, mRNA vaccines have demonstrated clear advantages in their rapid development speed and ability to induce robust immune protection, making this vaccine platform a strong contender among the various public health defense tools against future infectious disease outbreaks. Based on the current mRNA-LNP technology and low-sugar universal vaccine platform, Rock Bio has developed an innovative mRNA vaccine through the modification of the glycosylation sites on the SARS-CoV-2 spike protein. Compared with wild type control, the S2 glycosite deleted mRNA vaccine, or a more refined glycosite mutation variant in a subdomain of S2 called stem, offered broad protection against different SARS-CoV-2 variants and even other related coronaviruses such as MERS and SARS-CoV-1. Importantly, the vaccine induced very robust T cell response, which is now the most important indicator for long term protection. It thus has the potential to offer not only broader but also longer protection.

To build upon such an exciting technology, we are advancing our RBM-003 program, a low sugar universal mRNA vaccine against influenza. What differentiates this program from those undertaken by competitors in this space is our unique combination of glycoengineered mRNA sequences, and proprietary novel dendritic cell targeting LNPs. Thanks to its strong potential impact on public health and profound significance in the technology development, this program was awarded the inaugural Moderna Taiwan mRNA Innovation Award in 2023, making Rock Bio the only private enterprise to receive this prestigious honor that year.
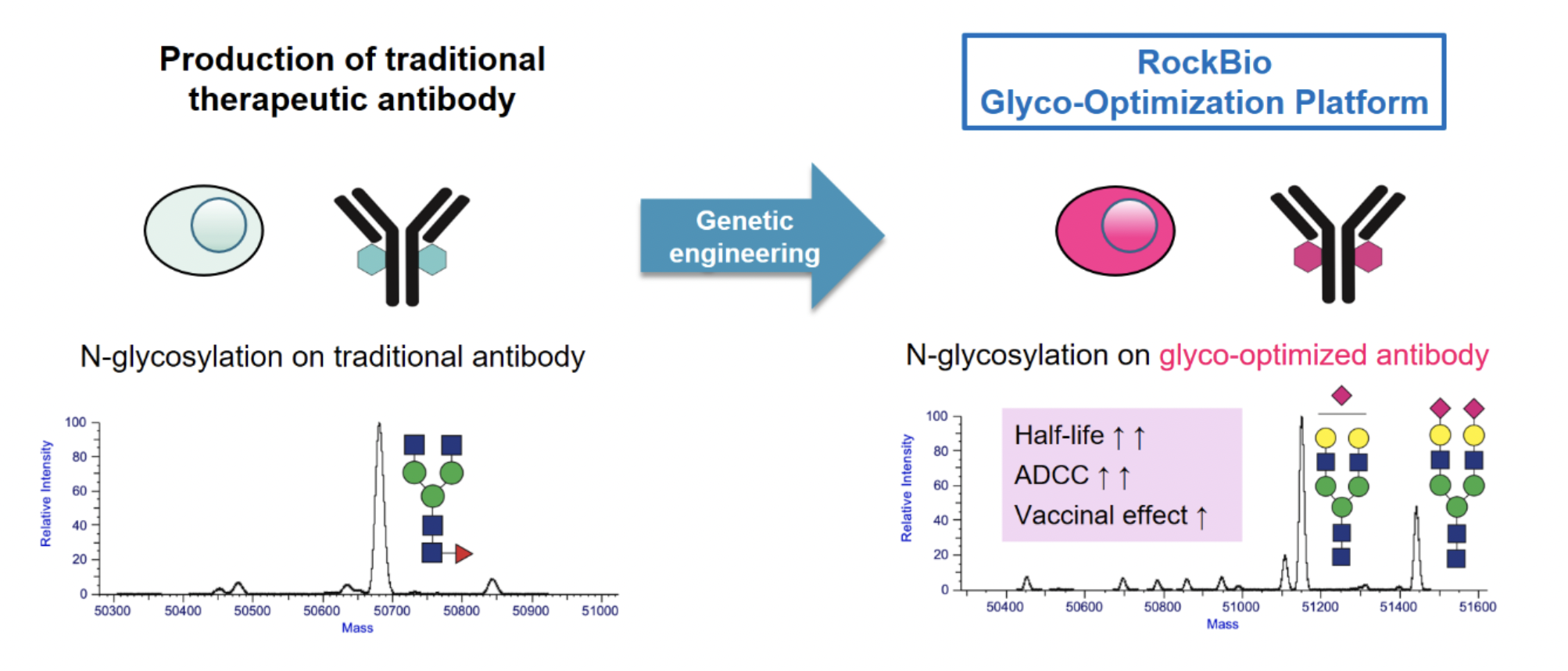
Another critical application of our glycoengineering technology lies in the production of improved protein biologics, such as therapeutic monoclonal antibodies. While the remarkable ability of monoclonal antibodies to identify and bind to target antigens is well-recognized, this is only part of their functionality. For therapeutic antibodies to be effective, they need to trigger a whole host of effector functions after antigen binding through interactions with a complex network of Fc receptors. This crucial interaction is highly dependent on the antibody's glycan profile.Commercial antibody drugs are most commonly produced from CHO cells, which result in glycan profiles that are suboptimal for their effector functions. Our proprietary glycoengineering technology addresses this issue through a dual strategy of genetic and medium engineering, enabling the production of recombinant antibody proteins with enriched, desirable glycan profiles, to improve efficacy and extend serum half-life. For biotech projects, this approach also offers the added advantage of generating new intellectual property, thereby extending product exclusivity for blockbuster antibody drugs that are nearing or have reached the end of their patent lives.